Future of Microbiome Science: Innovations Shaping What’s Next
Microbiome Innovations & The Future of Microbial Science: What’s Coming Next
The microbiome revolution is only at the beginning.
Scientists around the world now recognize the microbiome as one of the most influential systems in the human body, shaping immunity, metabolism, digestion, inflammation, oral health, mental health, and even aging.
The future of health, medicine, and personalized nutrition is deeply connected to advances in microbiome science.
If you missed the earlier parts of this cluster, start here:
👉 What Is the Human Microbiome? A Complete Guide to Microbes, Immunity & Digestion
👉 Oral Microbiota & Gut Health: How the Mouth Shapes the Entire Microbiome
👉 The Gut–Brain Axis: How Microbes Influence Mood, Stress & Appetite
👉 Microbiome Development From Birth to Adulthood
This final blog explores where microbiome science is heading — and how these innovations will shape the future of health.
Common Questions
1. What are next-generation probiotics?
Akkermansia, Christensenella, and SCFA-producing species like C. butyricum that influence gut lining, inflammation, and metabolism.
2. What is oral–gut microbiome therapy?
Therapy targeting the mouth first, improving gut health by modulating oral bacteria.
3. How do polyphenols support microbiome health?
They increase beneficial microbes, reduce inflammation, and promote mucosal immunity.
4. Are microbiome therapies becoming personalized?
Yes — precision nutrition and microbiome sequencing are shaping future interventions.
5. Does the microbiome influence brain health?
Absolutely. Microbes affect neurotransmitters, vagus nerve signaling, and stress response.
6. What’s the future of microbiome research?
Mucin-targeted therapeutics, oral–gut therapies, SCFA biology, next-gen probiotics, and sustainable microbiome innovations.
1. Next-Generation Probiotics (NGPs)
Traditional probiotics (Lactobacillus, Bifidobacterium) support digestion, but next-generation strains go deeper, influencing:
-
mucosal immunity
-
metabolic balance
-
inflammation reduction
-
gut barrier strengthening
-
oral–gut signaling
Examples include:
• Akkermansia muciniphila
Supports mucin regeneration & gut lining integrity.
• Christensenella species
Associated with healthy weight regulation, metabolic balance, and gut stability.
• Clostridium butyricum
Produces butyrate to fuel colonocytes and reduce inflammation.
Research shows these “keystone species” influence entire microbial ecosystems — not just their own niche.
2. Oral–Gut Therapeutics
Future microbiome therapy begins in the mouth, not the stomach.
The oral–gut axis plays a central role in:
-
inflammatory signaling
-
digestive enzyme activation
-
vagus nerve communication
-
microbial migration
-
upper-GI immune activity
📚 Reference:
Oral–Gut Microbiome Interaction — Frontiers (2021)
👉 https://pmc.ncbi.nlm.nih.gov/articles/PMC8125773/
Emerging evidence shows that targeting oral microbiota improves downstream gut health more effectively than capsule-based probiotics.
This is where chewable microbiome formulations will dominate—because they engage oral microbes before nutrients reach the gut.

3. Mucin-Layer Repair & Gut Barrier Immunology
The mucin layer is the first barrier between the body and the microbial world.
Novel research is focusing on:
-
mucin-stimulating microbes
-
mucosal immunology
-
epithelial repair
-
leaky-gut prevention
-
gut barrier-targeted nutrition
Key components include:
-
Akkermansia (mucin regeneration)
-
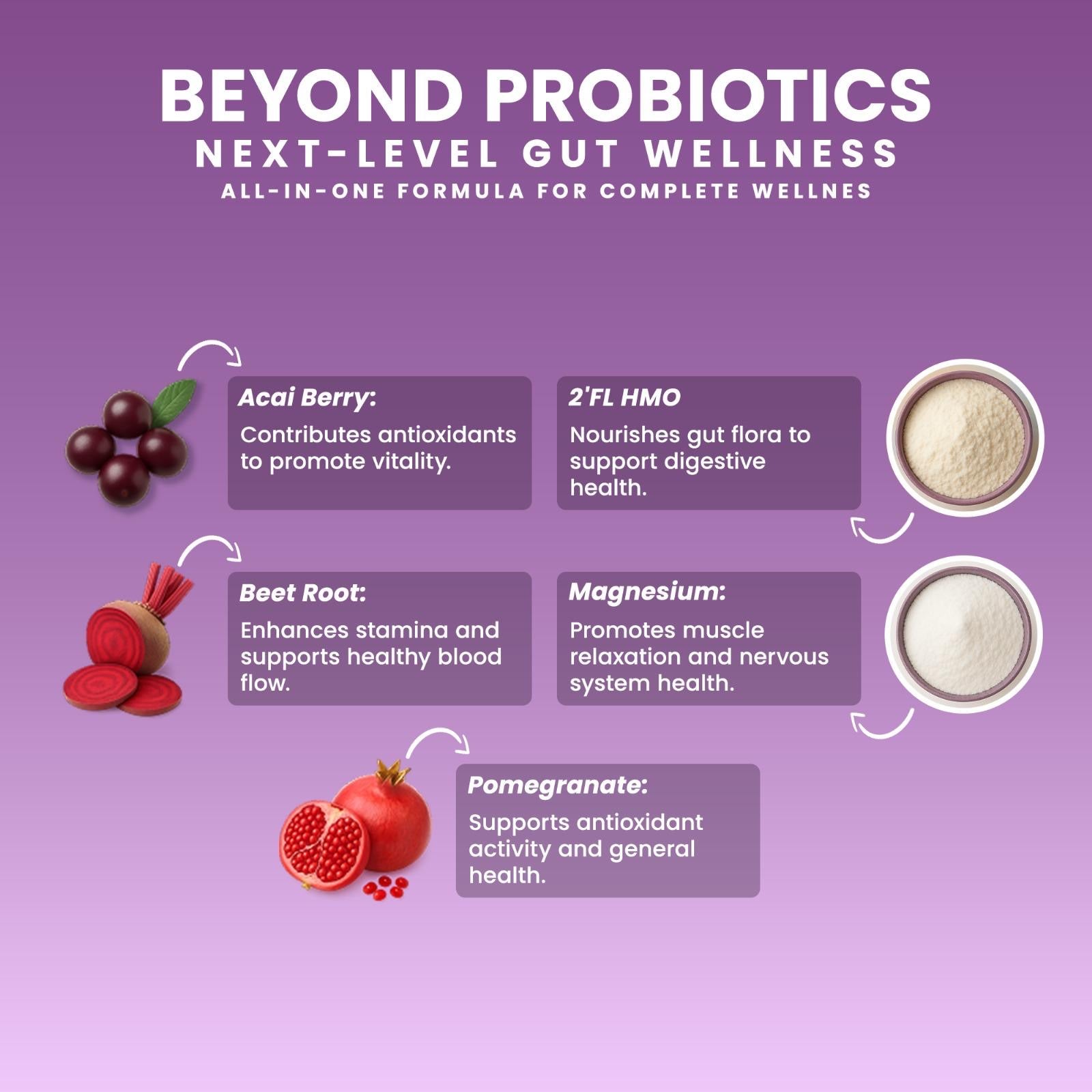
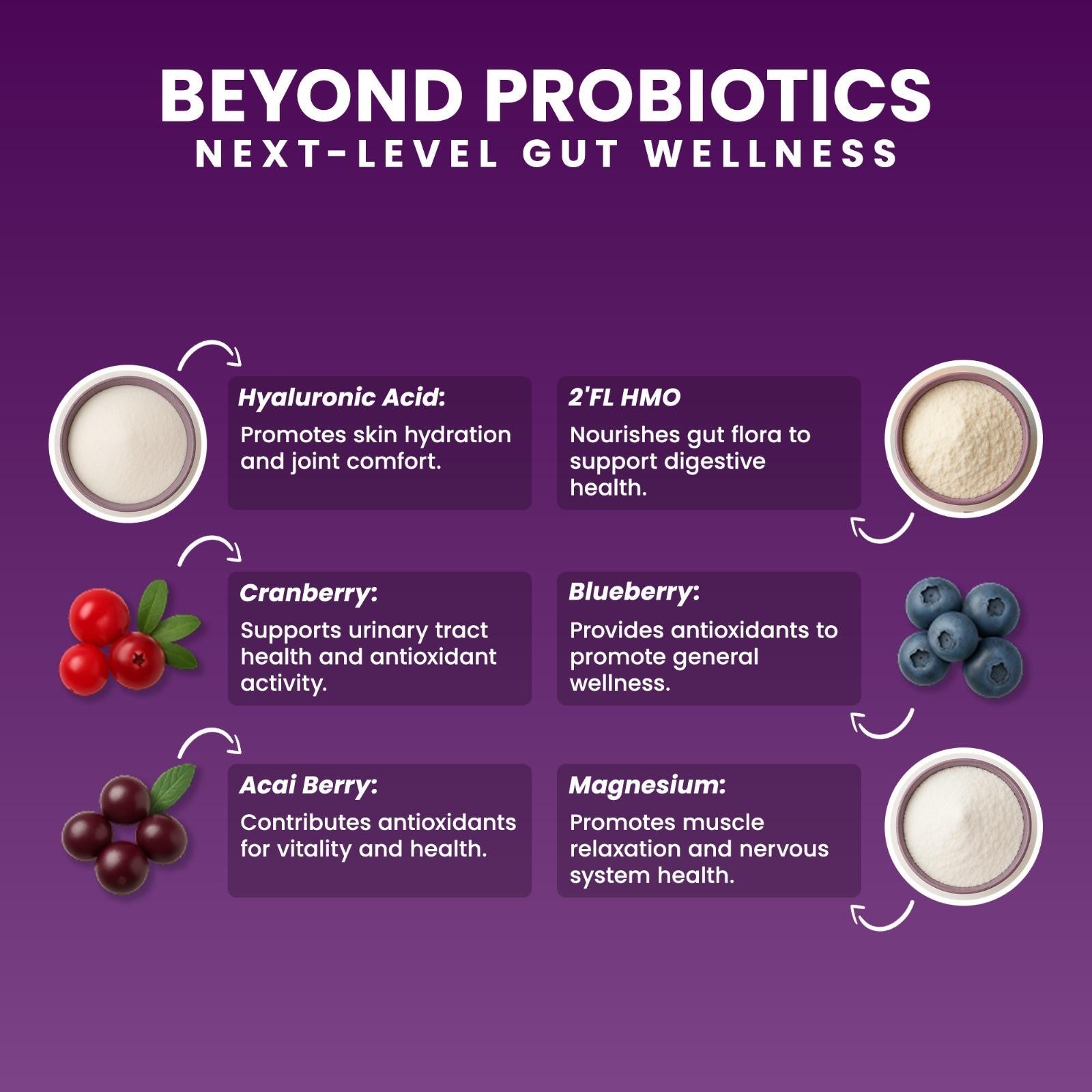
HMOs (2’-FL) (mucosal nourishment)
-
Butyrate (epithelial fuel)
📚 HMOs Reference — AJCN
👉 https://pmc.ncbi.nlm.nih.gov/articles/PMC6164445/
📚 SCFA Reference — C. butyricum
👉 https://pmc.ncbi.nlm.nih.gov/articles/PMC8078720/
Future therapeutics will center on restoring gut barrier strength — the foundation of systemic health.
4. Polyphenol–Microbiome Signaling (Postbiotic Precision)
Polyphenols do more than reduce inflammation — they act as microbial signaling molecules.
Polyphenols:
-
feed beneficial bacteria
-
stimulate mucin production
-
alter gene expression in microbes
-
increase Akkermansia
-
reduce harmful bacteria
📚 Polyphenols & Microbiota — Wang et al., 2022
👉 https://pmc.ncbi.nlm.nih.gov/articles/PMC9220293/
Future products will combine polyphenols + HMOs + SCFA-supporting strains for enhanced synergy.

5. Microbiome-Based Precision Nutrition
The future of nutrition is personalized, driven by microbiome profiling.
Doctors and nutritionists will use stool, saliva, and metabolite tests to:
-
personalize diets
-
recommend targeted probiotics
-
predict disease risk
-
optimize metabolic health
-
modulate inflammation
AI models will soon be able to predict microbial needs with stunning accuracy.
6. Microbiome Interventions for Mental Health
The gut–brain axis is an emerging field with extraordinary potential.
Microbes influence:
-
serotonin production
-
dopamine balance
-
stress resilience
-
anxiety and mood
-
cognitive performance
📚 Gut–Brain Axis — Physiological Reviews
👉 https://journals.physiology.org/doi/abs/10.1152/physrev.00018.2018
Future therapeutics will include:
-
psychobiotics
-
vagus-nerve modulators
-
SCFA-enhancing formulas
-
oral–gut–brain microbiome therapies
7. Microbiome Innovations for a Sustainable Future
Microbiome science is expanding beyond human health into:
-
agriculture
-
soil regeneration
-
environmental detoxification
-
food production
-
climate resilience
📚 Microbiome Innovations for a Sustainable Future — Nature Microbiology
👉 https://www.nature.com/articles/s41564-020-00857-w
Understanding microbial ecosystems will reshape industries — not just medicine.
✍️ Written by Ali Rıza Akın
Microbiome Scientist, Author & Founder of Next-Microbiome
Ali Rıza Akın is a microbiome scientist with nearly 30 years of experience in biotechnology and translational research in Silicon Valley. He is the discoverer of Christensenella californii, a novel human-associated microbial species linked to mucosal and metabolic health.
His scientific expertise spans:
• mucosal immunology
• oral–gut microbiome interactions
• SCFA metabolism
• gut barrier biology
• next-generation probiotics (Akkermansia, Christensenella, C. butyricum)
• host–microbe signaling
• translational microbial therapeutics
Ali is the author of Bakterin Kadar Yaşa: İçimizdeki Evren (Live as Long as Your Bacteria) and a contributor to Bacterial Therapy of Cancer: Methods and Protocols (Springer).
As Founder of Next-Microbiome, he develops advanced synbiotic formulations — including the first chewable Akkermansia-supporting synbiotic — engineered to strengthen the gut lining and optimize the oral–gut–brain axis.